 click on figure for more details
click on figure for more details
The discovery of high-temperature superconductors by Bednorz and Müller was the most exciting event in solid-state physics for several decades and was followed by frantic experimental activity. One of the key questions concerned the structure of the new materials. Bednorz and Müller believed that the copper oxidation states were critical, but with X rays, it was difficult to determine the oxygen coordination of coper in the presence of heavy metals such os yttrium and barium.
This problem was ideal for neutron diffraction, and at the end of March 1987, several large neutron laboratories solved the structure of the 90K superconductor, where the most famous solid-state laboratories had failed earlier the same month using X rays.
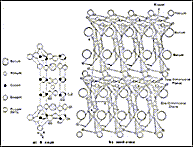
The figure below compares the structure of a 90K material YBa2Cu3O7, as obtained with X rays at Bell Laboratories and as obtained with neutrons by Institute Laue Langevin.
 click on figure for more details
click on figure for more details
The X ray and neutron structural models look quite different, but in fact both are correct. It is just that the oxygen atoms were not well located with X rays, and this implied a quite different picture of the structure.
It later became clear that the neutron model actually supports Bednorz and Müller's ideas, even though there are now no octahedra. Figure 1b shows that two-dimensional planes of copper oxide are connected together by one-dimensional chains of copper oxide via so-called " bridging" oxygen.
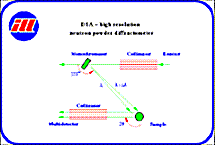
The different planes are separated only by the different d spacings between them, not only by their orientation. Different d spacings produce constructive scattering at different angles (sin theta/lambda), so the neutron diffractometer must have high angular resolution. This is obtained with fine Söller collimators in front of the detectors and monochromator.
The neutron beam from the reactor (shown below) is then defined by a Söller collimator and a particular wavelength lambda selected by a monochromating crystal. This monochromatic neutron beam is scattered by the powdered sample, and the intensity from the different crystal planes is measured by scanning a second Söller collimator in front of a detector through all scattering angles.
 click on figure for more details
click on figure for more details
Bednorz and Müller's ideas were spectacularly successful when they showed that perovskite(La,Sr)2CuO4 was superconducting up to 35K, much higher than had been thought possible until then. Theirs was the only theoretical idea that has ever led to the discovery of a new superconductor.
YBa2Cu3O7 has several disadvantages as a practical high-Tc superconductor, including the ease with which it can loose oxygen and the twinned nature of the crystals. A more stable phase, YBa2Cu4O8, with double CuO chains can be made under oxygen pressure Butcher et al. and van Eenige et al. have shown that with applied pressure, the Tc of YBa2Cu4O8 increases remarkably, from 80K to 108K Kaldis et al. have therefore used neutron powder diffraction to measure the changes in the Cu-O bond lengths as pressure increases.