Diamond 2.1: Powerful visualization
Diamond 2.1 Features Overview... Next:
Useful for all kinds of crystal structures...
This page gives you a survey of Diamond's capabilities for the graphical
representation of molecular or crystal structures.
Basic models
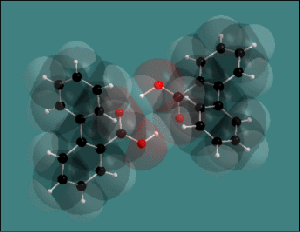
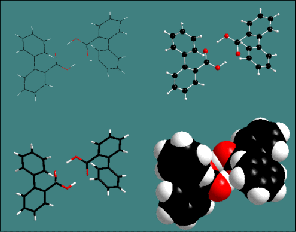
This illustration on the right compares four representations of the same
structure picture [1] using wire (upper left), sticks (lower left),
ball-and-stick (upper right), and space-filling model (lower right).
Both ball-and-stick and wire model can be superimposed with a transparent
space-filling-model:
Polyhedra and thermal ellipsoids
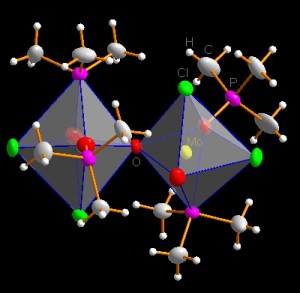
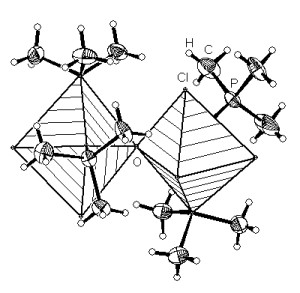
Basing on the standard model (the ball-and-stick model), anisotropic
displacement parameters can be visualized as thermal ellipsoids, and the
coordinations of atoms may be represented by coordination polyhedra. Polyhedra
and thermal ellipsoids can be mixed in one and the same picture! The left one
of the two pictures below shows a rendered representation with transparent
polyhedra, whereas the right one is a typical ORTEP-like representation showing
the axes of the ellipsoids, together with thick tapered bonds and shading edge
to enhance the three-dimensional impression [2]:
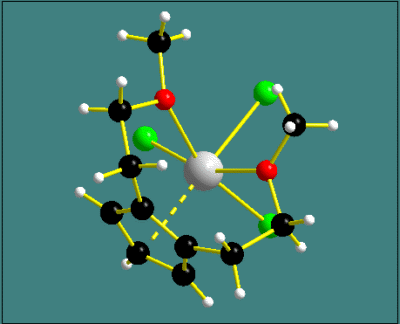
Fragmentated and broken-off bonds
One of the components of a bond's design is its fragmentation. If greater than
zero, this can be used e.g. to display delocalized bonds, like in the picture
on the right [3].
Broken-off bonds will be used to indicate that a picture has been cut out of a
bigger molecule or out of a polymeric framework. (There is a picture on the "Molecules
and Packing Diagrams" page).
References:
[1] Name: biphenyl-2-carboxylic acid
Authors: Dobson, Allison J., Gerkin, Roger E.
Title: Biphenyl-2-carboxylic Acid: a Layered Structure
Journal: Acta Cryst. (1998). C54, 795 - 798
[2] Name:
mu-Oxo-bis[dichlorooxo(trimethylphosphine-P)(trimethylphosphine
oxide-O)molybdenum(V)] diethyl ether hemisolvate
Authors: F. A. Cotton, L. M. Daniels and S. Herrero
Journal: Acta Cryst. (1999), C55, CIF Access Papers Section: IUC9900018
[3] Authors: van der Zeijden, Adolphus A. H., Mattheis, Chris,
Fröhlich, Roland
Title: [1,2-Di(methoxyethyl)-h5-cyclopentadienyl]trichlorozirconium(IV)
Journal: Acta Cryst. (1998). C54, 458 - 460
Diamond 2.1 Features Overview... Next:
Useful for all kinds of crystal structures...
|


